A new method to study protein complexes by chemical cross-linking
Researchers from the Aebersold group develop a complementary cross-linking chemistry that connects acidic residues in protein complexes.
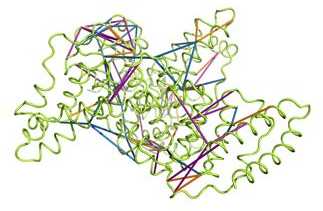
Chemical cross-linking is an emerging technique to study the architecture of protein complexes by forming covalent bonds between amino acid side chains and identifying the reaction products on the peptide level by mass spectrometry. Up to now, the technology has been dominated by reactions that connect the primary amine groups in lysine residues due to their higher reactivity. Alexander Leitner from the Aebersold group developed a complementary approach that connects the less reactive acidic residues Asp and Glu under conditions that are compatible with the integrity of protein complexes. In collaboration with researchers at the Max-Planck-Institute of Biochemistry in Martinsried, Germany, and at Stanford University, USA, the applicability to multisubunit complexes – the TRiC/CCT chaperonin and the 26S proteasome – was successfully demonstrated. Applying different cross-linking reactions in parallel provides more information for deriving low-resolution models of such large complexes. In addition, some complexes may not yield any cross-links with the conventional chemistry, but can be successfully studied with this novel method, as shown in a collaboration with researchers at the Forschungszentrum Jülich, Germany (Raasch et al., J. Biotechnology, in press. external pageDOIcall_made)
This work was featured by external pageNature Methodscall_made and external pageChemical & Engineering Newscall_made.
Reference: Leitner A, Joachimiak LA, Unverdorben P, Walzthoeni T, Frydman J, Förster F, Aebersold R. Chemical cross-linking/mass spectrometry targeting acidic residues in proteins and protein complexes. Proc Natl Acad Sci U S A. 2014 Jul 1;111(26):9455-60 external pageDOIcall_made.
